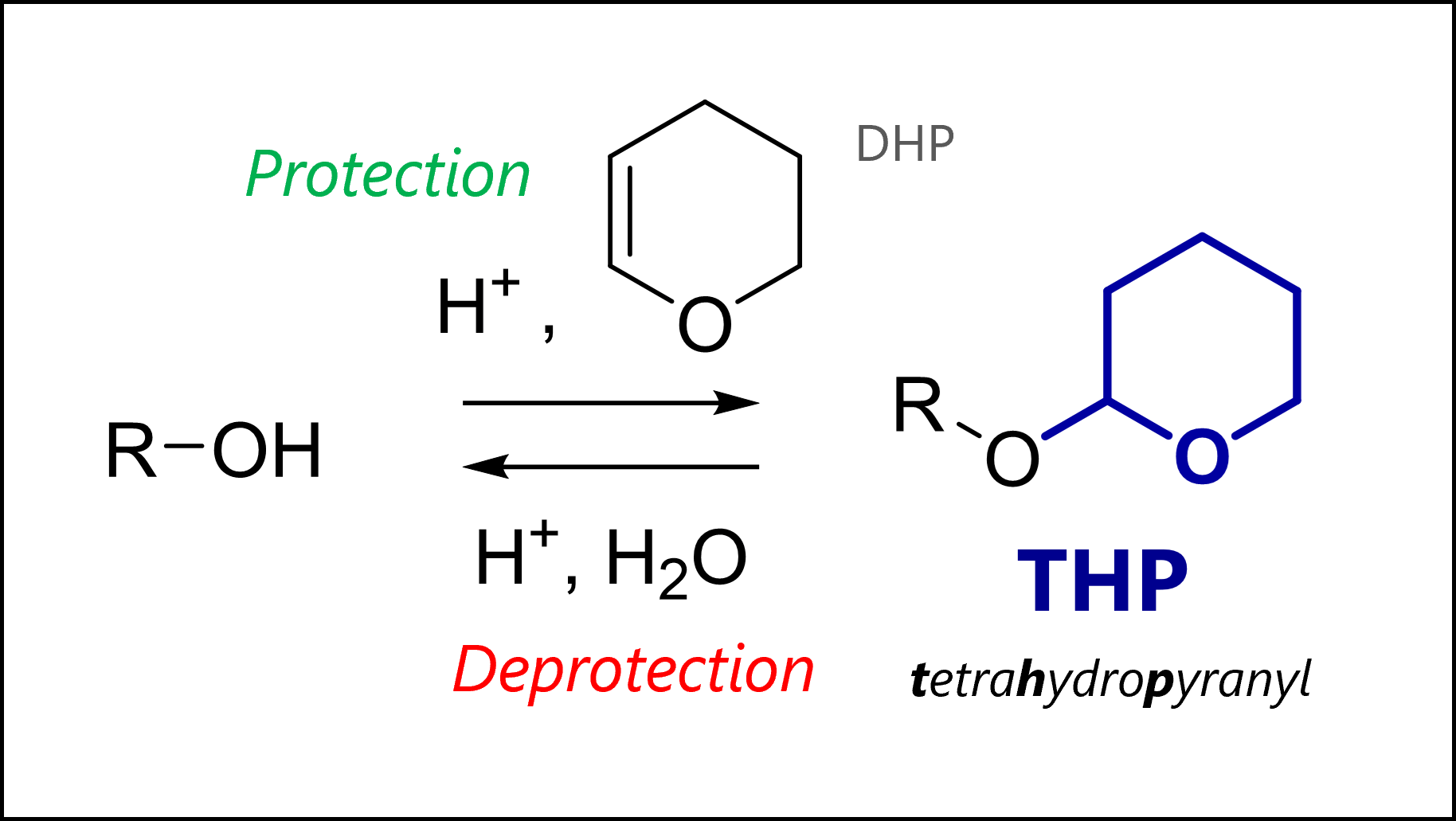
The THP protecting group protects alcohols as acetals. THP is introduced with DHP and acid, and removed through acidic solvolysis.
THP (tetrahydropyranyl) is a hydroxyl protecting group, but less common than silyl groups like TBS. ➡️ Here’s what you’ll learn:
👀 3D model of THP. It’s essentially a beefy MOM!
What is the THP Protecting Group?
Tetrahydropyranyl ethers were one of the first protecting groups for alcohols. Nowadays, they are seen less commonly, though still used. The THP group is easily removed under acidic conditions (mechanism below) and stable to organometallic nucleophiles, electrophiles (as the protected oxygen is less nucleophilic), reduction or base. The protected THP ethers are actually a type of acetal (‘double-ether’).
THP Protection Mechanism

THP protection uses acid catalysis and 3,4-dihydro-2H-pyran or DHP. The mechanism proceeds by THP pre-activation with acid, leading to a stabilized cation. Here, the oxonium is drawn but you can imagine the other resonance form with the positive charge on the carbon which is ultimately where the ion is most electrophilic.
Our free hydroxyl group then attacks the carbon in a nucleophilic addition, and loses a proton to give the protected THP ether. The last step regenerates our acid catalyst.
The most common protection conditions are catalytic TsOH or pyridinium p-toluenesulfonate (PPTS, a form of TsOH with lower acidity) together with 3,4-dihydro-2H-pyran in dichloromethane.
THP Deprotection Mechanism
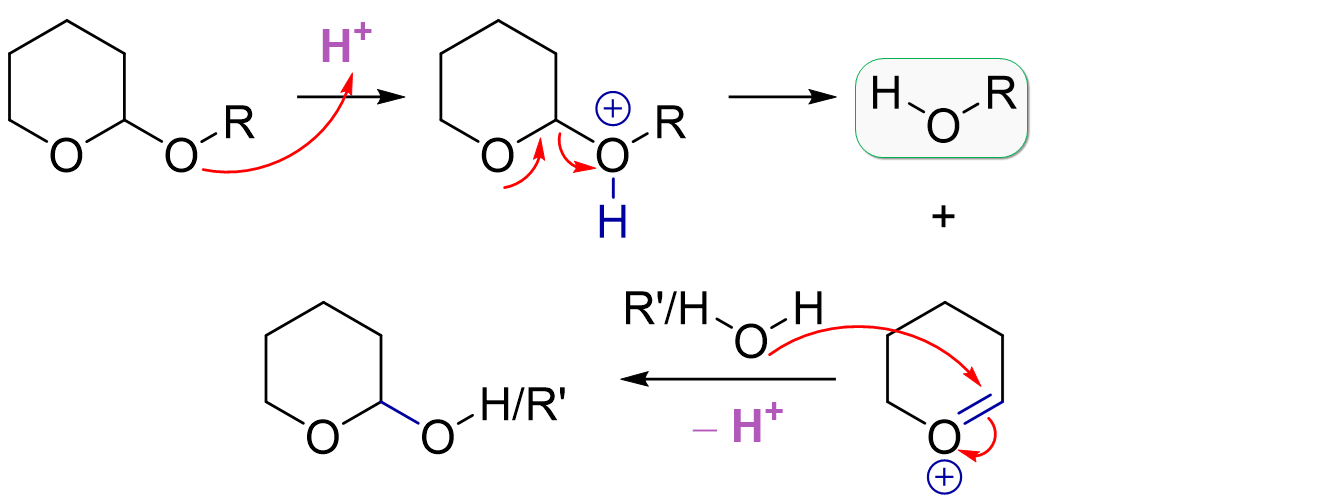
THP deprotection is similar to MOM deprotection and actually also similar to THP protection. Acid catalysis activates the acetal system towards dissociation of our initially protonated alcohol. Again, it’s the same stabilized cation intermediate but based on the choice of solvent used, we have different potential byproducts. The solvent is obviously present in large excess, so it will preferentially attack the carbocation instead of our just liberated hydroxyl group. For example, methanol gives the methyl-substituted THP ether while use of water would give the free hydroxyl group (this can open to the linear aldehyde).
The most common deprotection conditions are AcOH:THF:H2O or PPTS in EtOH.

Tired of serious chemistry?
Take a break with “Periodic Tales – The Freshman Mole”, a satirical novel that’s the opposite of educational.
Dedicated to every chemistry and STEM student who asked: “Why did no one warn me?”
THP protecting group diastereomers

One of the drawbacks of the THP protecting group versus the TBS protecting group, beyond its lower stability, is that it introduces a second chiral center.
If our starting material has already at least one chiral carbon, we form diastereomers. This can complicate the separation and identification (e.g., NMR) of products – because as you know, diastereomers have different physicochemical properties.
Interestingly, some older research [1] tried to make use of this ‘drawback’. In this work, the chemists used a THP-derivative as a chiral auxiliary for nucleophilic additions to an aldehyde in the molecule.

In these derivatives, one side of the aldehyde is shielded from nucleophilic attack while the other is exposed. This leads to very high diastereoselectivity at the newly formed carbon (a tertiary alcohol). It’s not terrible useful but interesting that a protecting group can be used to exert diastereoselectivity. You could imagine this potentially being useful in some complicated total syntheses.
Thanks for reading! Check out other protecting group lessons or my educational videos if you are interested!
THP protection experimental procedure [2]
“To a 100-mL, single-necked, round-bottomed flask equipped with a magnetic stir-bar, argon inlet with septum, was charged with dihydropyran (1.5 equiv), followed by CH2Cl2 (15 mL) and PPTS (177.4 mg, 0.706 mmol, 0.1 equiv). The contents were cooled to 0 °C in an ice-bath. Then a suspension of iodobenzyl alcohol 9 (2.08 g, 7.06 mmol) in CH2Cl2 (10 mL) was added at 0 °C over 10 min. After addition, the contents were warmed to rt. Dihydropyran (0.97 mL, 10.59 mmol, 1.5 equiv) was added again to the mixture after 30 min, because the starting material was still observed by TLC analysis. After another 30 min of stirring, H2O (50 mL) was added and the mixture was extracted with CH2Cl2 (3 x 50 mL). The organic layers were combined and washed with brine (2 x 50 mL), dried with Na2SO4, filtered and the solvent was removed under reduced pressure by rotary evaporation. The crude material was further purified using column chromatography (SiO2, 70 g; hexanes/EtOAc, 3:1) to afford (2.67 g, >99%) THP ether 26 as a colorless wax.”
THP deprotection experimental procedure [3]
“To a solution of alkene 12 (38.6 mg, 0.047 mmol) in 2-propanol (0.95 mL), p-toluenesulfonic acid monohydrate (21.7 mg, 0.114 mmol) was added at 0 °C and stirred for 17 h at room temperature. The reaction mixture was diluted with water, extracted with dichloromethane, washed with brine, and dried over sodium sulfate. The residue was purified by thin layer chromatography (hexane/ethyl acetate = 5/1). Alcohol 13 (34.6 mg, quant.) was obtained as a colorless oil.”
THP Protecting Group References
- P. G. M. Wuts, T. W. Greene: Greene’s Protective in Organic Synthesis (Wiley)
- [1] The tetrahydropyranyl group as a chiral auxiliary for the nucleophilic addition to α-alkoxy ketones | André B. Charette , Abdel F. Benslimane , Christophe Mellon | Tetrahedron Letters 1995, 36, 8557
- [2] Total synthesis of (+)-papulacandin D | Scott E. Denmark, Tetsuya Kobayashi, Christopher S. Regens | Tetrahedron 2010, 66, 4745
- [3] Total Synthesis of Eutyscoparol A and Violaceoid C | Takatsugu Murata, Takuto Iwayama, Teppei Kuboki, Shotaro Taguchi, Shou Tsugawa, Takumi Yoshida, Hisazumi Tsutsui, Ayana Shimauchi, Yukiho Kosaka, Isamu Shiina | Asian Journal of Organic Chemistry 2024, 13, e202400148

Leave a Reply
You must be logged in to post a comment.