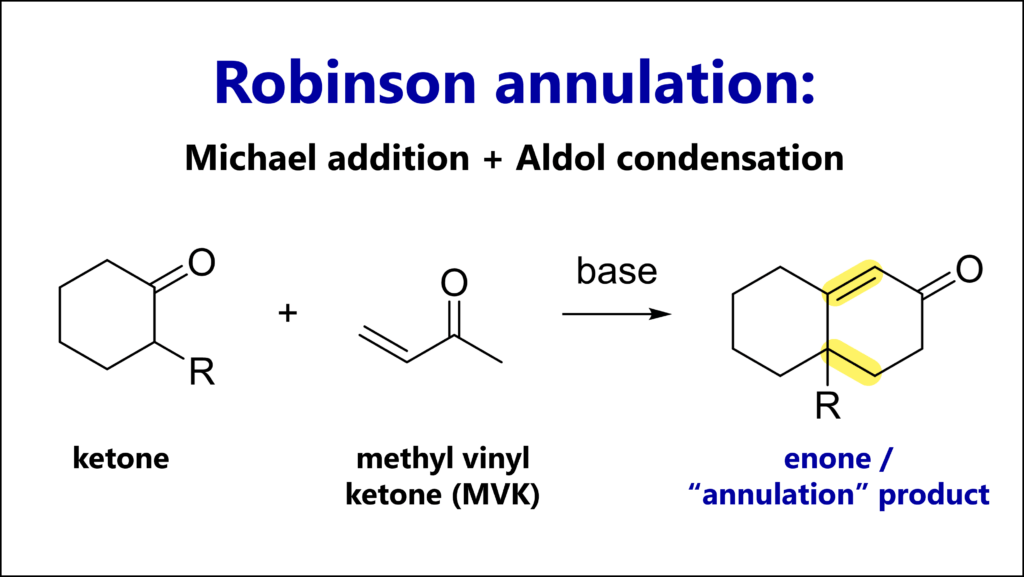
The Robinson annulation is a cyclization reaction consisting of Michael addition of a ketone to an unsaturated ketone, and an Aldol condensation, giving bicyclic unsaturated enones.
🫡 What you’ll learn in this Name Reaction article:
what are the two starting materials for a robinson annulation?
The Robinson annulation requires an enolizable ketone (Michael donor) and an α,β−unsaturated ketone (Michael acceptor), typically methyl vinyl ketone (MVK).
The last important component is catalytic base (or less often, acid). To achieve all sub-reactions in one step, stoichiometric base is often used.
Robinson annulation mechanism
The Robinson annulation is a C-C bond forming reaction, and also a cyclization reaction (by the way, an annulation is simply the fusion of a new ring to a molecule through two new bonds).
The mechanism consists of three steps:
1) Michael addition, also called conjugate or 1,4-addition
2) Intramolecular Aldol addition
3) Dehydration to give the enone ring, making 2) + 3) an Aldol condensation)
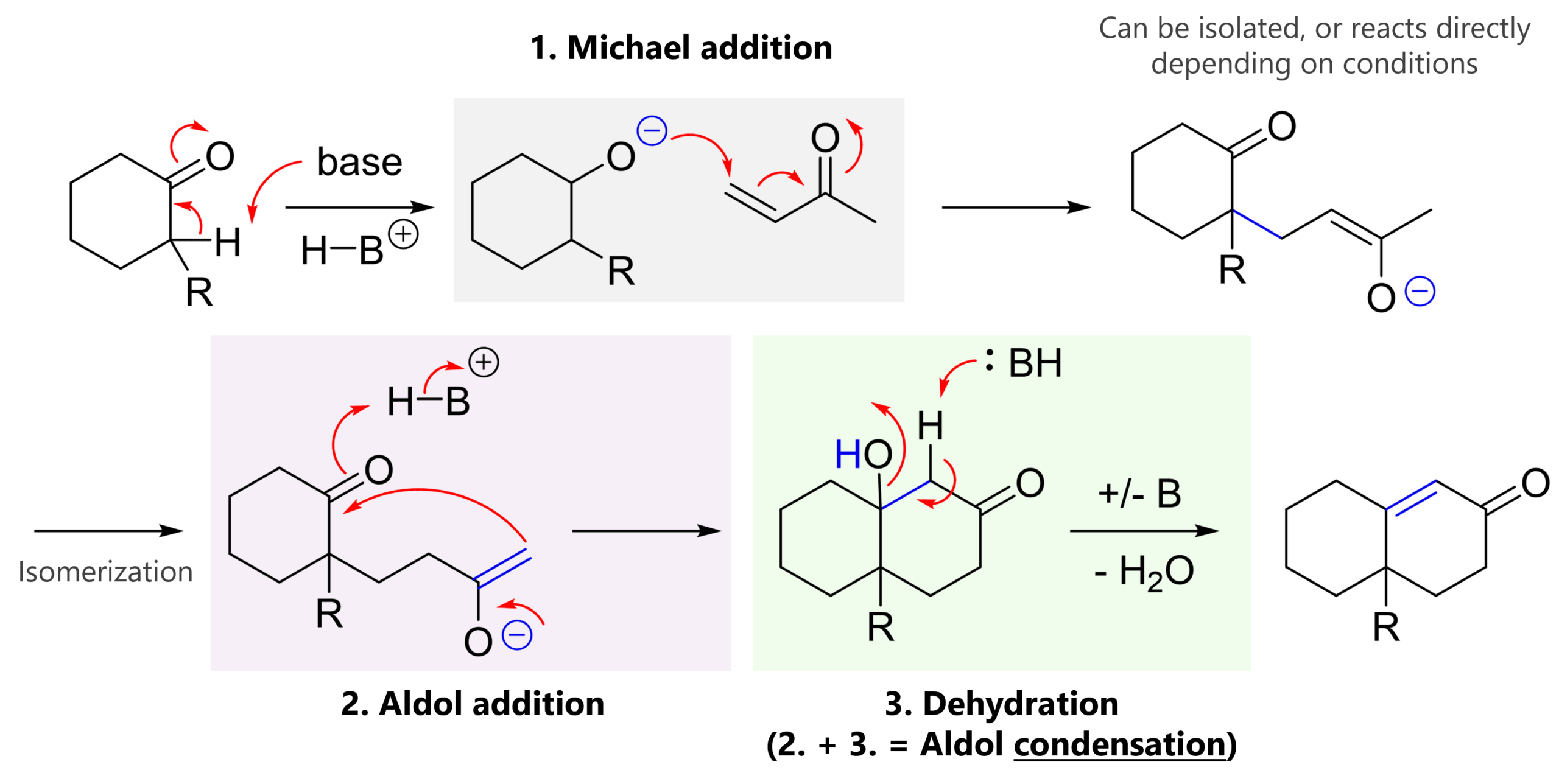
The first step is deprotonation at the alpha-position of the carbonyl (here, cyclohexanone) to give an enolate. Remember, this position is relatively acidic due to its resonance stabilization. This is why we need base catalysis!
Note: Starting material and conditions can influence on which side the enolate forms (e.g., think R = -CO2Me vs. tBu). Know your thermodynamic vs. kinetic enolates!
This enolate is a nucleophile, so it attacks our electrophile (here methyl vinyl ketone). This forms one of the two carbon-carbon bonds.
Depending on the specific case, we can either i) isolate this product and then perform the Aldol condensation, or ii) the product continues directly. The easiest way to draw this is to simply assume that the product isomerizes to the reactive enolate (you can also draw two intermediate steps where the enolate is first protonated, and then again deprotonated from the other side).
You will know that intramolecular reactions are very efficient (pre-organization and proximity of reactive partners). The Aldol reaction is an attack to our internal ketone, forming the second carbon-carbon bond.
The 3D model helps visualize how the side chain can orient itself in a way where the nucleophilic enolate gets close to the electrophilic ketone.
Finally, water is eliminated (E1cb) to give the conjugated enone product. This makes steps 2 and 3 an Aldol condensation. This final step can require more forcing conditions (low temperatures are fine for Michael and Aldol additions, but not always for elimination).
Robinson annulation Watchouts
- The reaction is useful if the starting material ketone and enone are simple (because we would get fewer side reactions / higher yield) or readily available (because we would care less about yield). To manage complex cases, chemists have developed modifications of the reaction.
- Methyl vinyl ketone can polymerize and thus, other “MVK equivalents” have been developed for practical reasons (see later section)
- The reaction can be done in one step or alternatively two (isolation of the Michael addition product). Note that our initial Michael addition product, does not immediately cyclize to the ketone. This would form a four-membered ring which is much less favored than the six-membered ring that can be formed once the other enolate is formed.
- The reaction is typically base-catalyzed, but there are also examples of acid-catalyzed variants (e.g., H2SO4).
- We can use the carbonyl and double bond in the product for further reactions which is why people call such groups “synthetic handles”. The reaction has been widely used, particularly for the synthesis of steroids.
Robinson Annulation conditions
As mentioned, the Robinson annulation often uses catalytic amounts of base. For the one pot reaction, commonly used bases are hydroxide bases (e.g., KOH) or alkoxide bases (e.g., NaOMe, KOtBu). Sometimes, stoichiometric use of stronger hydride bases (e.g., NaH) is used. The stepwise method often uses different conditions, like a cat. hydroxide base for Michael addition, followed by an amine base like piperidine for the intramolecular Aldol condensation.
For exemplary reaction conditions, please see the experimental procedures at the end of the article.

Tired of serious chemistry?
Take a break with “Periodic Tales – The Freshman Mole”, a satirical novel that’s the opposite of educational.
Dedicated to every chemistry and STEM student who asked: “Why did no one warn me?”
History of the robinson annulation
Before we get into examples, just some historical context. I hope you will not be shocked that the Robinson annulation was developed by the British organic chemist Robinson. Together with Rapson, they reported the method for the first time in 1935 [1]. They performed the reaction in a step-wise manner, using sodium azide (NaNH2) as the base.
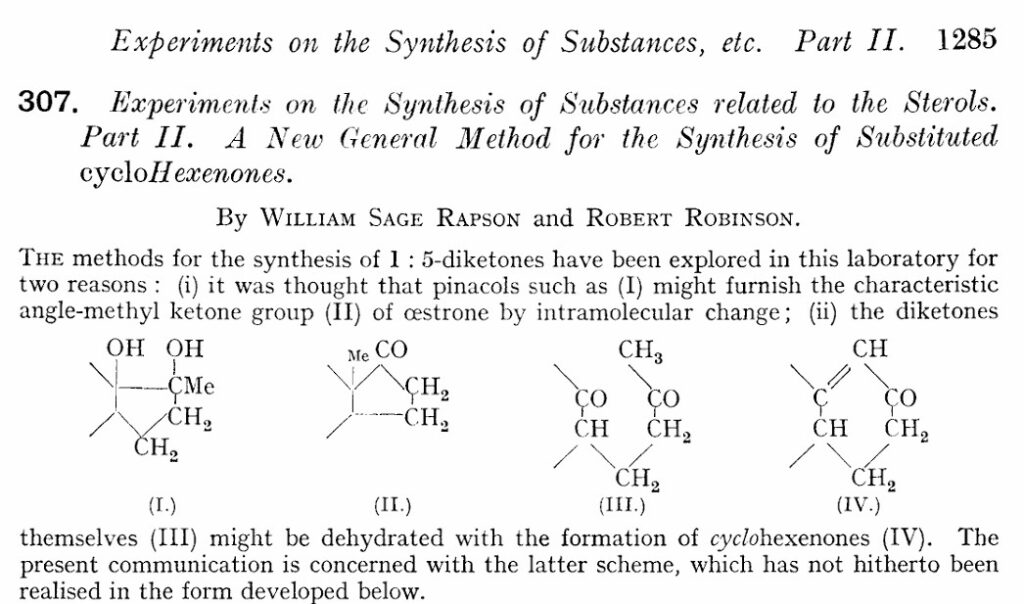
Robinson is well known for finding a one-pot synthesis of tropinone, inventing the use of curly arrows to show electron movement in reaction mechanisms, structural determinations like morphine, penicillin and strychnine. He received the Nobel prize in 1947.
Here’s a fun story: Robinson reported the total synthesis of cholesterol using his annulation reaction in 1951 – around the same time as R. B. Woodward – the OG of organic chemistry.
In Name Reactions, Fourth Edition, Jie Jack Li wrote: Here is a story told by Derek Barton about Robinson and Woodward.
“By pure chance, the two great men met early in a Monday morning on an Oxford train station platform in 1951. Robinson politely asked Woodward what kind of research he was doing these days; Woodward replied that he thought that Robinson would be interested in his recent total synthesis of cholesterol. Robinson, incensed and shouting ‘Why do you always steal my research topic?’, hit Woodward with his umbrella.”
—An excerpt from Barton, Derek, H. R. Some Recollections of Gap Jumping, American Chemical Society, Washington, D.C., 1991.
robinson annulation examples
Let’s get into three concrete examples.
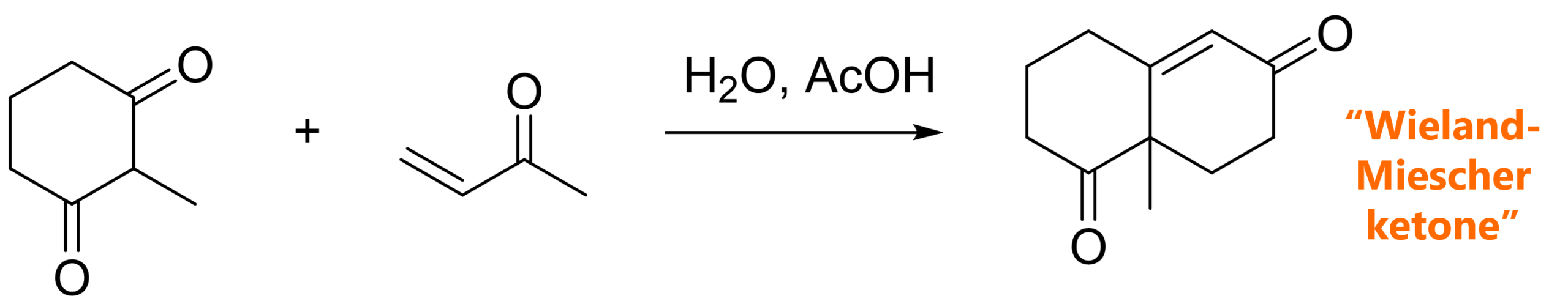
#1 The first example is so famous that the product even got its own name – the Wieland-Miescher ketone [2]. This is a case of a Robinson annulation under acidic conditions.
#2 In this next example, the R group is just an ethyl ester. This obviously makes the reaction very efficient because the starting material is a great Michael donor / easily enolizable.
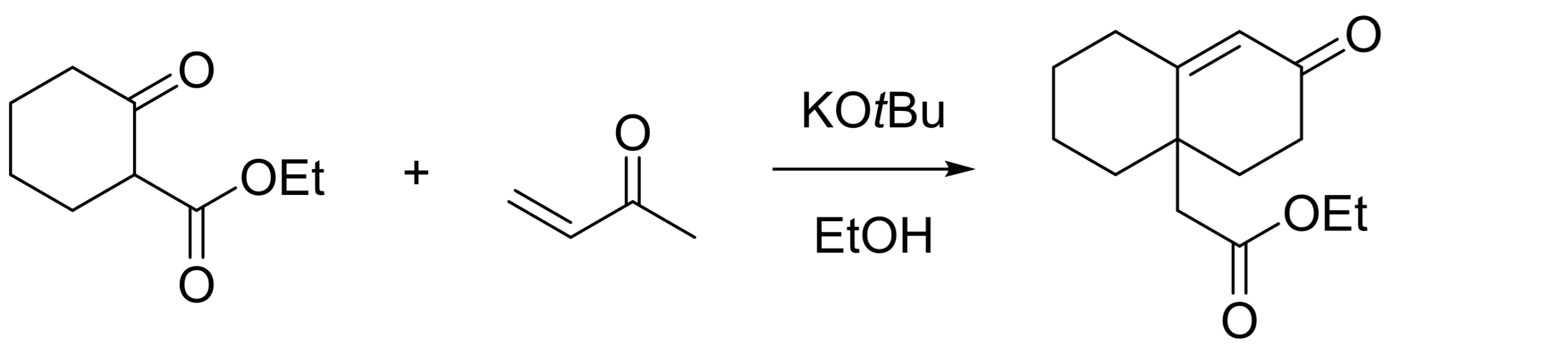
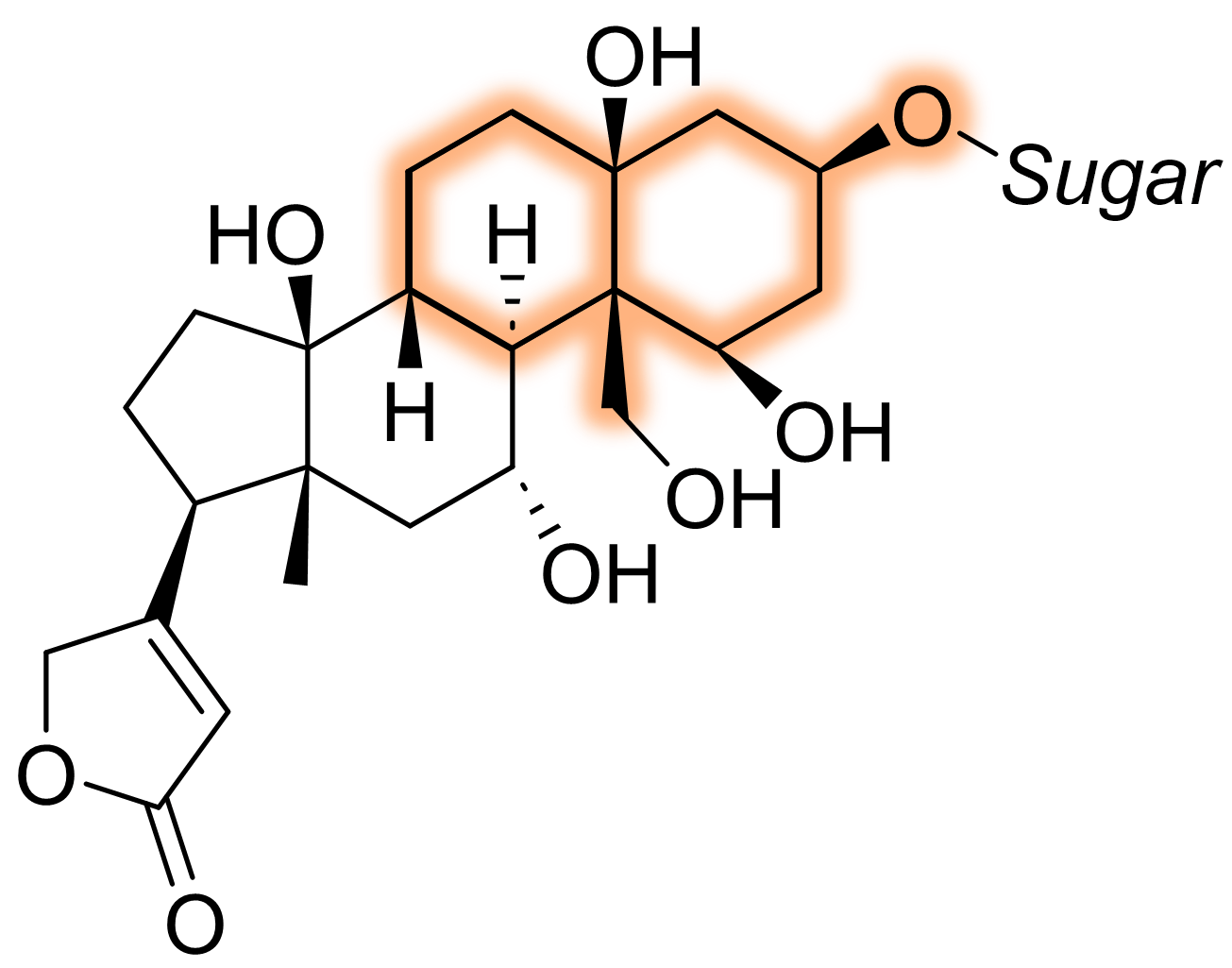
The product was intended to be used for the synthesis of ouabain, a glucoside steroid [3]. You can quickly see where the 6/6-bicyclic ring would fit.
#3 What about this next example – can you explain the formation of the product if our starting material has a sulfinyl group? [4]
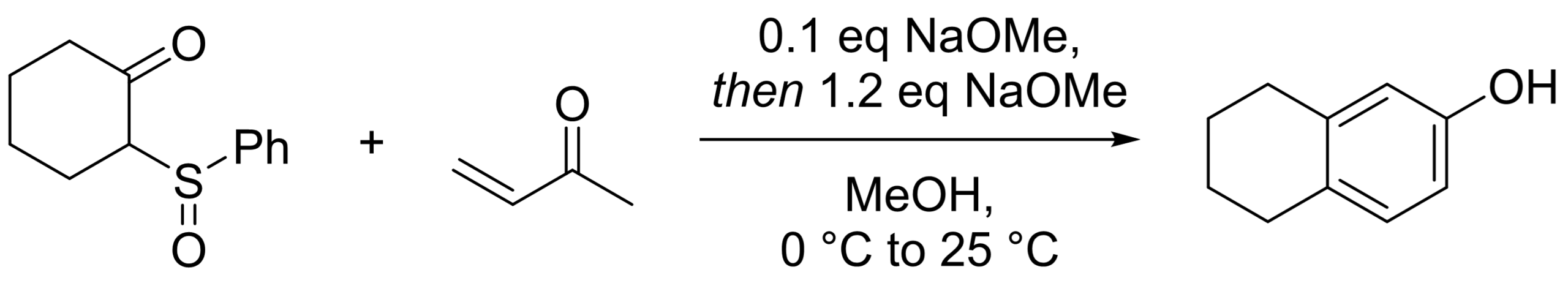
We obviously form a bicyclic annulation product as expected, but we also lost the sulfinyl group. This is because after Robinson annulation, elimination of phenyl sulfenic acid gives cyclohexadienone which can aromatize to the phenol.
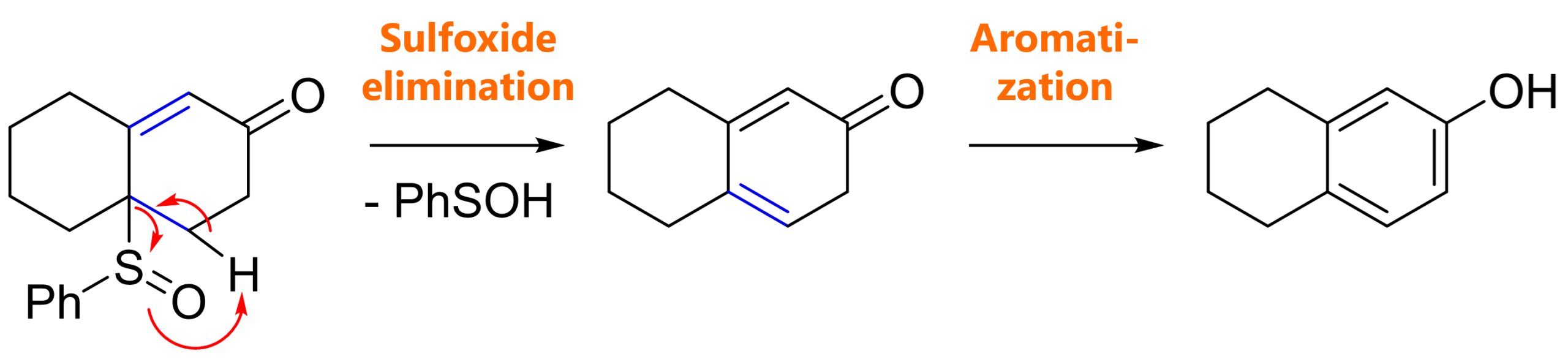
robinson annulation with different michael acceptors
How does the above differ versus our next example [5]? This is actually from a 1950 report of the legendary R. B. Woodward!
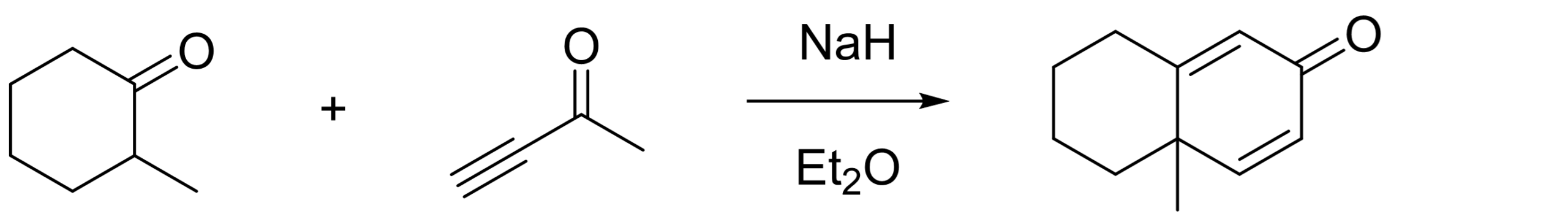
Now, our Michael acceptor is an alkynone with a triple bond. This means that after the Michael addition, we’re left with a double bond (instead of the usually remaining single bond). The Aldol condensation happens as expected. Without any other substituent on the ring, you’d also expect enolization of the ketone group to the phenol (as we saw in example #2). However, we have a methyl group in our starting material, so the aromatization is impossible!
robinson annulation with Methyl vinyl ketone equivalents
As already mentioned, enones like methyl vinyl ketone can suffer from polymerization and other side reactions. Precursor compounds like β-chloroketones or β-ammoniumketones can be used instead of enones [6].
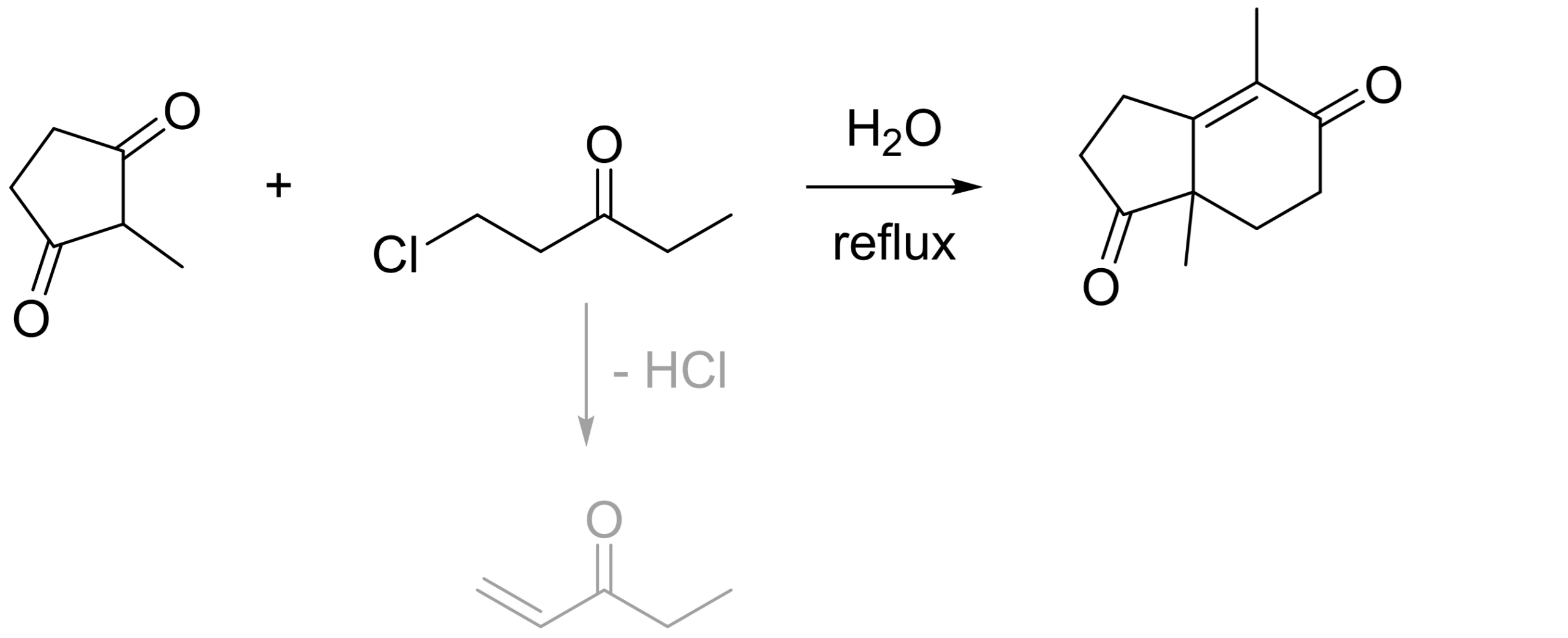
In this case, the HCl generated from the β-chloroketone acts as the catalyst for the Michael addition and Aldol reaction. So, this is again an acid-catalyzed example.
Stereoselective robinson annulation reactions
Like in most reactions, we can achieve stereoselective product formation with the right setup. There are two areas to explore: enantioselectivity and diastereoselectivity.
The first logic is to use a chiral base to get enantioselectivity of one product over another one. The amino acid L-proline does just that! [7]
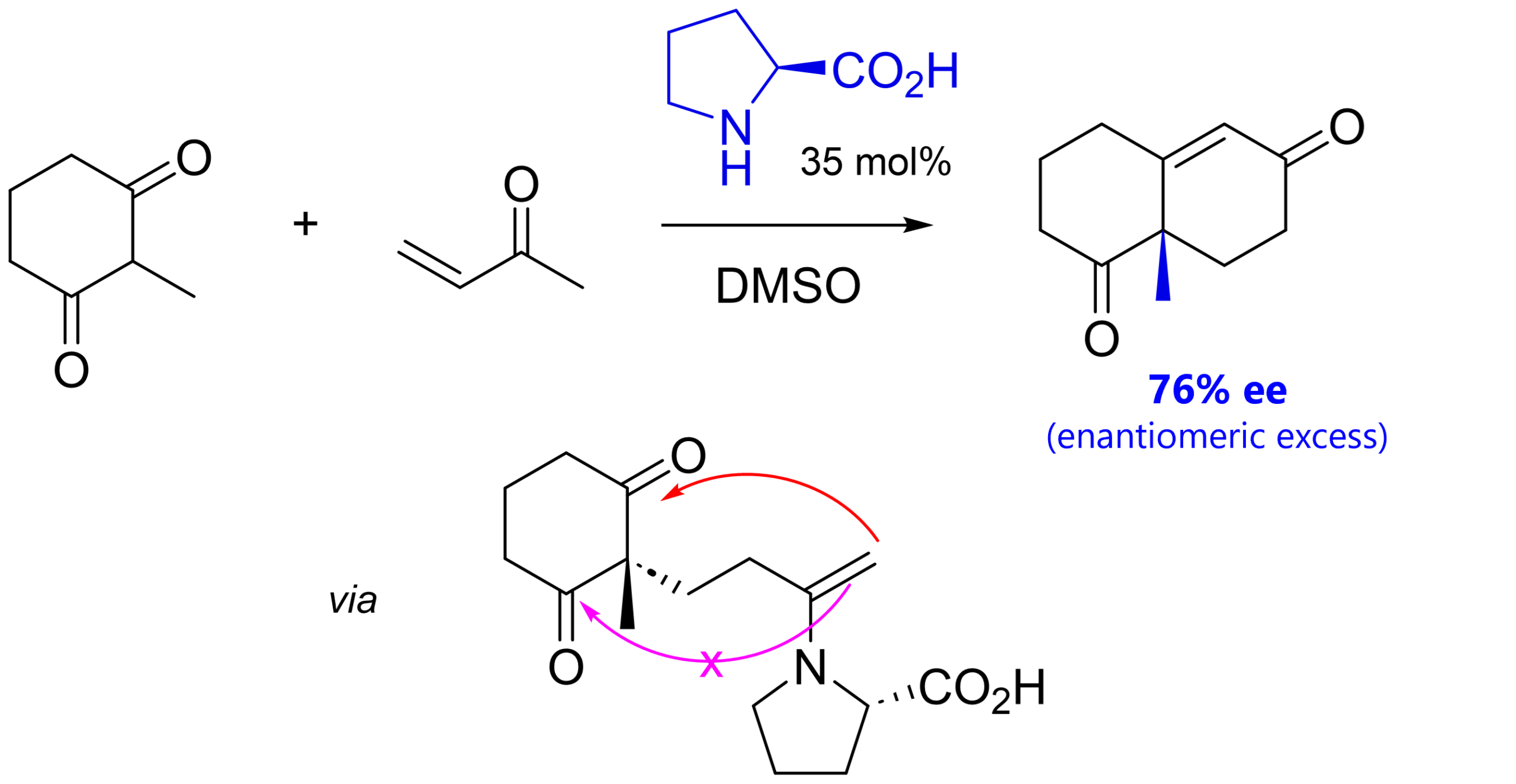
This enantioselectivity comes from the fact that, after Michael addition (which is also accelerated by proline), proline forms a chiral enamine prior to Aldol condensation. Due to proline’s chirality, attack on one of the two pro-chiral ketones is favored (there have been different transition state models proposed which you can look up if you’re interested [8]).
Interestingly, it’s not just the stereochemistry of proline that plays a decisive role. Using a reduced proline, where the carboxylic acid is reduced to the alcohol, does not facilitate the final dehydration under the same conditions. The carboxylic acid can act as an intramolecular proton donor, making the elimination more efficient.
Diastereoselective robinson annulation
The second way that stereoselectivity can happen is through diastereoselectivity. This happens when our Michael acceptor and/or Michael donor come with additional substituents themselves. The two new C-C bonds can now be formed with some stereochemical bias (the explanation always depends on the specific case – e.g., kinetic / sterics vs. thermodynamic explanation).
Here’s an example [8] where where our Michael donor has an ester group (nothing new!), and also our Michael acceptor has a silyl substituent in the β-position. In the product, both substituents end up at sp3-hybridized / tetrahedral carbons so we can expect different diastereomers forming. The observed ratio was 6:1 in favor of the cis-substituted product.
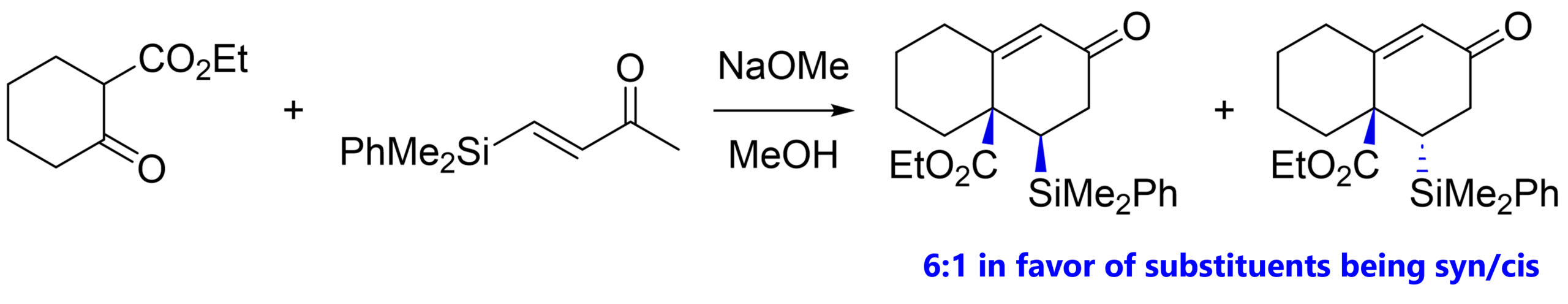
Thermodynamic control might explain the major diastereomer, as it allows the bulky silly group to occupy the equatorial ring position.
Functionalization of Robinson annulation products
We mentioned above that the annulation products can be pretty handy. This is an opportunity for you to test your organic reaction toolkit. What reactions do you know that might be applied to that bicyclic enone system?
Here are some examples: nucleophilic addition, reduction, epoxidation, cyclopropanation – and even oxidation.
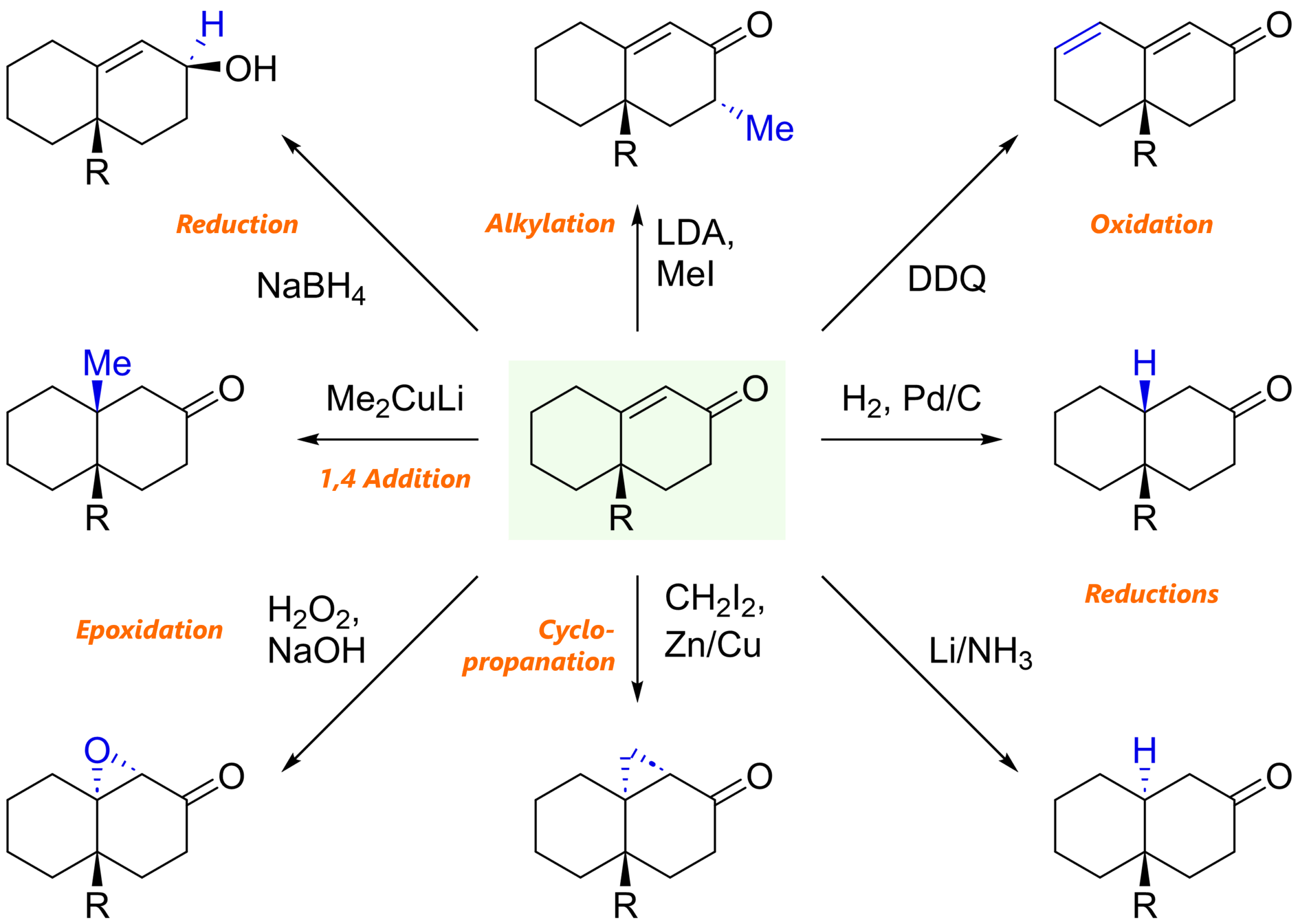
That’s it for this article! Feel free to check out other articles on my page or my educational videos!
Robinson annulation experimental procedure [2]
“Potassium t-butoxide (296 mg, 2.65 mmol) was dissolved in ethanol (EtOH, 25 mL) at 0 °C, under argon. After stirring for 20 min, ethyl 2-oxocyclohexanecarboxylate (8 mL, 50 mmol) was added slowly at the same temperature. After 15 min at 0 °C, methyl vinyl ketone (4.15 mL, 50 mmol) was added over 5 h via syringe pump. Then the resulting deep-orange solution was heated to reflux and kept for 6 h. The reaction mixture was cooled to ambient temperature. After stirring for 18 h, the mixture was poured into a separatory funnel containing saturated NH4Cl (30 mL) and extracted with Et2O (3 x 200 mL). The combined organic layers were dried over MgSO4 and solvent removal afforded 14 (11.0 g) as a crude orange oil, which was used without further purification.
Robinson annulation experimental procedure [3]
“A solution containing 2-(phenylsulfinyl)cyclohexanone (1.97 mmol, 439 mg)and sodium methoxide (0.21 mmol, 0.10 mL of 2.15 M methanolic NaOCH3) in 10mL of absolute methanol cooled to 0 °C under nitrogen was treated dropwise (20 min) with methyl vinyl ketone (2.76 mmol, 193 mg, 0.24 mL, 1.4 equiv) and the reaction mixture was stirred at 0 °C for 23.5 h. Additional sodium methoxide (2.36 mmol, 1.10 mL of 2.15 M methanolic NaOCH3) was added and the mixture was allowed to warm to 25 °C where it was stirred for 31 h. The resulting reaction solution was poured onto 5% HCl (25 mL) and the aqueous phase was extracted thoroughly with ether (5x 10 mL). The combined etheral layers were washed with saturated NaCl, dried (MgSO4),and concentrated in vacuo. Chromatography (10 to 25% ether-hexane gradient elution) afforded 168mg (291 theoretical, 58%) of pure phenol as a white solid.”
Robinson annulation experimental procedure [7]
“A solution of L-proline (0.32 g, 2.8 mmol) and 2-methyl-1,3-cyclohexadione (1 g, 7.7 mmol) in 50 mL of anhydrous DMSO was stirred under argon at 35 °C until the ketone and proline were completely dissolved. To this solution, freshly distilled methyl vinyl ketone was slowly added dropwise (0.99 mL, 11.9 mmol). The reaction was vigorously stirred at this temperature for 89 g and then quenched with ethyl acetate/saturated ammonium chloride. The organic layer and aqueous layer were separated with an addition of brine. The aqueous phase was filtered, and evaporated in vacuo. Crude product was purified by column chromatography.”
Robinson annulation References
- [1] Experiments on the synthesis of substances related to the sterols. Part II. A new general method for the synthesis of substituted cyclohexenones l | W. S. Rapson; R. Robinson | J. Chem. Soc., 1935, 1285
- [2] Über die Herstellung mehrkerniger Ketone | P. Wieland; K. Miescher | Helv. Chim. Act. 1950, 33, 2215
- [3] First Synthesis of the A/B Ring of Ouabain | M. E. Jung; G. Piizzi | Org. Lett. 2003, 5, 137
- [4] A simple and convenient phenol annulation | D. L. Boger; M. D. Mullican | J. Org. Chem. 1980, 45, 5002
- [5] Synthesis and Rearrangement of Cyclohexadienones | R. B. Woodward; T. Singh | J. Am. Chem. Soc. 1950, 72, 494
- [6] Robinson annelation by reactions of 2-methyl 1,3 diketones with a .beta.-chloro ketone | P. A. Zoretic; B. Bendiksen; B. Branchaud | J. Org. Chem. 1976, 41, 3767
- [7] A proline-catalyzed asymmetric Robinson annulation reaction | T. Bui, C. F. Barbas III | Tetrahedron Letters 2000, 41, 6951
- [8] Proline-Catalyzed Direct Asymmetric Aldol Reactions | B. List; R. A. Lerner; C. F. Barbas III | J. Am. Chem. Soc. 2000, 122, 2395
- [9] Synthetic Approach to the AB Ring System of Ouabain | M. E. Jung; G. Piizzi; J. Org. Chem. 2003, 68, 2572

Leave a Reply
You must be logged in to post a comment.