Links to detailed articles for each protecting group below (mechanisms, etc.)!

Protecting groups are temporary shields for reactive functional groups to prevent side reactions and maximize chemoselectivity of reactions. Protecting groups are critical to enable multi-step synthesis of highly functionalized targets like natural products or peptides.
Pic: AI illustrating a chemist & a protecting group xD
Like death and taxes, protecting groups have become a consecrated obstruction which we cannot elude; we will continue to depend on them for the foreseeable future and we can admire the ingenuity that is invested in their design, but it is a wise practitioner who holds that “protection is not a principle, but an expedient”.
P. J. Kocieński [1]
Why do we need Protecting Groups?
Multiple functional groups can have similar reactivity. In chemistry, we typically want to modify a single functional group while preserving the others. However, the most reactive site might not be the one we want to do our next chemistry with!

For example, ketones as well as aldehydes react electrophilically at the carbonyl carbon. You will know that aldehydes are inherently more reactive than ketones.
Unless we can chose to introduce functional groups sequentially and avoid interference through some sort of perfect synthetic design (see more below), protection becomes vital.
Requirements for protecting Groups
In a dream world, protective groups deliver on all of these requirements:
- Protection should proceed selectively, safely and in good yield
- The protecting group should be stable to the projected synthetic plan (duh)
- Deprotection should proceed selectively, safely and in good yield
- Side products from introduction or cleavage should be easily separable and not react with the product or starting material
- As an extension of #2-3, the protecting group should be orthogonal to other protecting groups required throughout the synthesis (see below)
- The protecting group should be as simple as possible. It should lack additional reaction sites, molecular complexity (e.g., not form stereogenic centres), and should be easy to characterize
Evidently, there is no universally “best protecting group” that fulfils all criteria for all cases. Thus, chemists have amassed a large armamentarium (finally I can use this word) of complementary protecting groups. For instance, a boatload of different flavours of alcohol protecting groups are available.
Also, all protective groups come with some downsides. In many cases, we are willing to tolerate and work around them. For example, many protecting groups create highly reactive intermediates during their deprotection. The use of scavenger molecules is essential to prevent additional side reactions.
Orthogonality of Protective Groups
The concept of orthogonality refers to two or more protecting groups which can be removed in any order with specific reagents or conditions that do not affect other protecting groups (or to a minimal degree).
We will illustrate orthogonality (or lack thereof) when talking about specific protective groups.
To Protect or Not Protect, That is the Question
As we will see, protecting groups can be cool and teach us some things about chemistry. However, they also distract chemists. We require two steps to introduce and remove them, making our synthesis’ economy worse. They’re a just necessary means to an end, not a goal per se.
In response – as the science of chemistry evolved – there has been increasing focus on protecting-group-free syntheses since the early 2000s [2]. While highly complex targets have been synthesized since [3] – some of them will be covered here – the field remains critical knowledge for every chemistry student and practitioner.
Protecting Group List
This page will summarize key characteristics of the most important protecting groups in organic synthesis.
As the number of posts will be slim initially, a more comprehensive table can be found below.
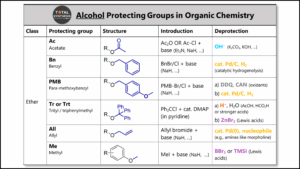
- Alcohol Protecting Groups in Organic Chemistry
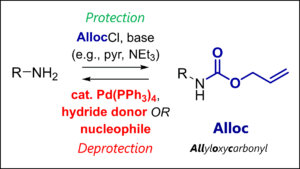
- Alloc Protecting Group: Alloc Protection & Deprotection Mechanism
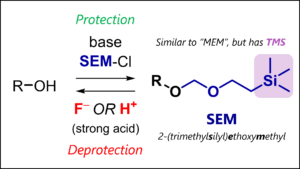
- SEM Protecting Group: SEM Protection & Deprotection Mechanism
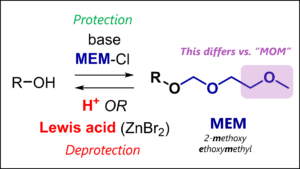
- MEM Protecting Group: MEM Protection & Deprotection Mechanism
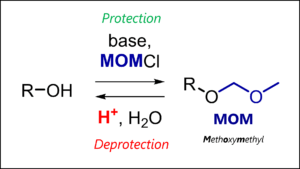
- MOM Protecting Group: MOM Protection & Deprotection Mechanism
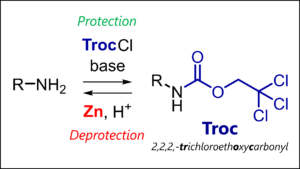
- Troc Protecting Group: Troc Protection & Deprotection Mechanism
Carbonyl protecting groups:
– Acetals (e.g., 1,3-dioxane)
– Thioacetals
Hydroxyl protecting groups:
– Silyl ethers (e.g., TMS, TBDMS, TIPS)
– Esters (e.g., acetyl, benzoyl, pivaloyl)
– Benzyl ethers (e.g., benzyl, PMB, ONB…)
– Acetals (e.g., MOM, THP, MEM, SEM)
Amine protecting groups:
– Carbamates (e.g., Boc, Fmoc, Cbz, Alloc, Troc, Teoc)
– Amides (e.g., acetyl)
– Sulfonamides (e.g., tosyl, nosyl)
Carboxylic acid protecting groups:
– Esters (e.g., methyl, benzyl, allyl, t-butyl)
– Silyl esters (e.g., TBDPS, TIPS)
– Orthoesters
[1] P. J. Kocienski, Protecting Groups (2005), Thieme
[2] I. S. Young, P. S. Baran, Nature Chem. 2009, 1, 193 | Protecting-group-free synthesis as an opportunity for invention
[3] C. Hui, F. Chen, F. Pu, J. Xu, Nature Reviews Chemistry 2019, 3, 85 | Innovation in protecting-group-free natural product synthesis