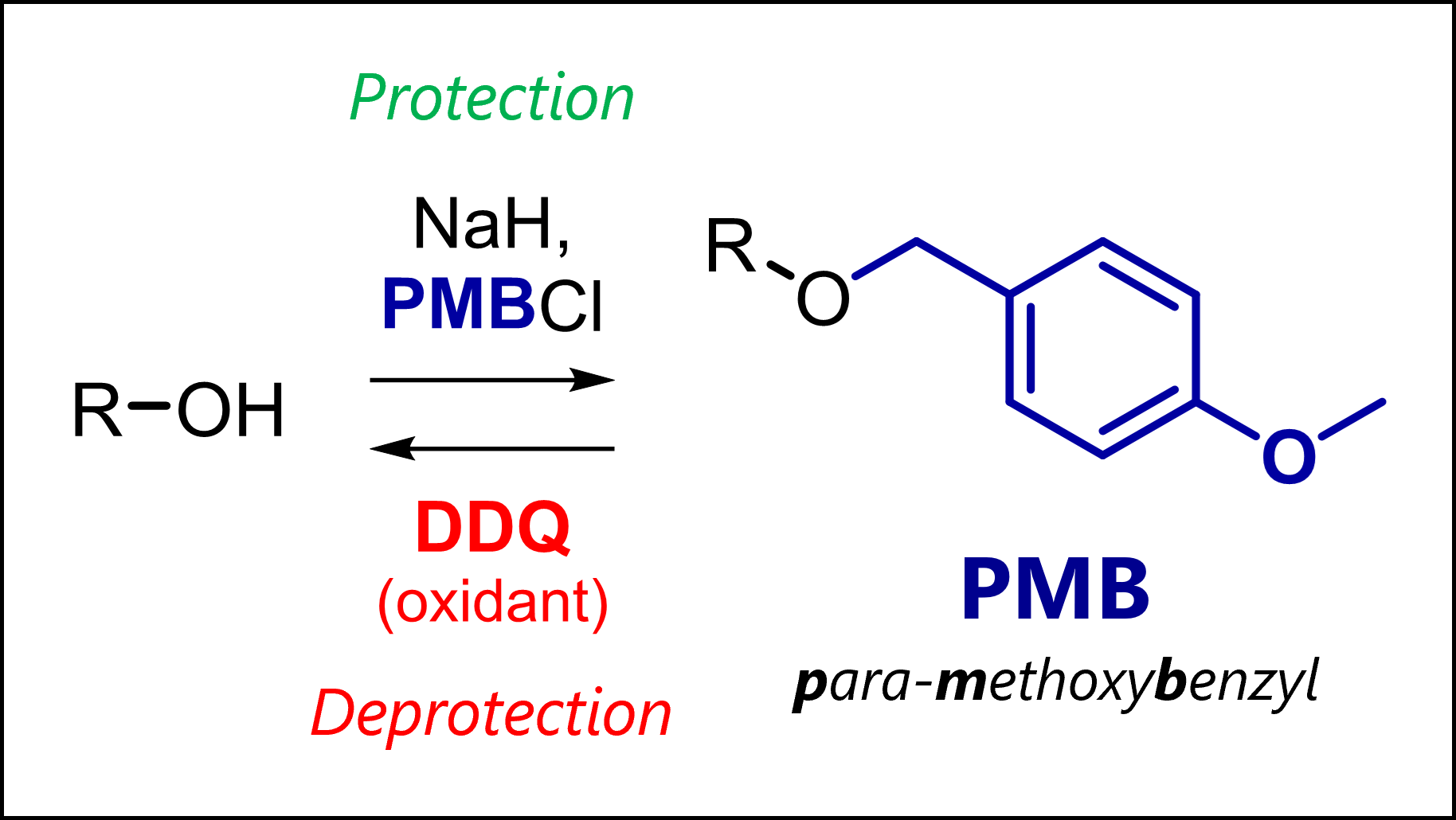
The PMB protecting group protects alcohols as less reactive ethers in organic synthesis. PMB is deprotected oxidatively or with strong acids.
🫡 Here’s what you’ll learn here:
👀 Left: 3D model of the PMB group to help you visualize it.
What is the PMB Protecting Group?
PMB is a para-methoxybenzyl group, introduced by Yonemitsu in 1982 [1] to protect alcohols and other nucleophilic functional groups. Although PMB ethers are less stable to acid than normal benzyl ethers, they can uniquely be cleaved oxidatively (see below). This allows selective deprotection protocols which becomes critical in complex organic synthesis.
PMB Protection Mechanism

The main method of PMB protection is the Williamson ether synthesis. This reaction uses a moderately strong base to generate an alkoxide which undergoes SN2 substitution with an activated agent like PMB-Cl. Typical conditions include sodium hydride NaH in THF/DMF or DMSO. However, stronger bases like nBuLi work as well.
Beyond PMB-Cl, the reagent you will see most, there are also other methods of PMB protection. Beyond halide variants like PMB-I or PMB-Br, PMB-trichloroacetimidate with catalytic acid can also protect hindered tertiary alcohols. This is due to its higher reactivity. The PMB-pyridyl thiocarbonate with silver(I) is another example.

Interestingly, tetrabutylammonium iodide can be used catalytically for sluggish reactions as well. Here’s a question for you: Do you know how that catalysis works?
PMB DeProtecTION Mechanism WITH DDQ

This protective group differs from others in that it undergoes easy single electron transfer (SET) with DDQ (2,3-dichloro-5,6-dicyano-l,4-benzoquinone).
The electron-donating methoxy group stabilizes intermediary radical and oxonium ion. Normal benzyl protecting groups oxidize as well, but much slower than PMB. Obviously, this is due to the O-PMB methoxy group.
After some proton exchanges, water captures the carbocation. As with every redox reaction, the electrons removed from O-PMB (oxidation) end up in the reduced hydroquinone product. Compared to the quinone in DDQ, this system is aromatic.
The hemiacetal formed after water addition can fragment to give the deprotected hydroxy – as well as anisaldehyde. One drawback of is unintended side reaction of the aldehyde or intermediary PMB cations with nucleophilic functional groups, as well as polymerization. Thus, it is common to add nucleophilic scavengers (e.g., thiols) that capture these reactive species. This is a common thread for some deprotections (e.g., Boc).
By the way, other oxidants like cerium(IV) ammonium nitrate (CAN) or NBS might work when DDQ fails.

Tired of serious chemistry?
Take a break with “Periodic Tales – The Freshman Mole”, a satirical novel that’s the opposite of educational.
Dedicated to every chemistry and STEM student who asked: “Why did no one warn me?”
PMB protecting group Orthogonality
The DDQ reduction (typically 1.1-1.5 equivalents DDQ in dichloromethane-water mixtures) leaves several functional groups and other protecting groups (MOM, THP, TBS, Bz…) alone. This makes PMB an interesting orthogonal protecting group.
However, electron-rich groups like dienes or trienes can be unintended victims of DDQ. This makes complete sense: electron-rich groups are nucleophiles and thus like to react with oxidants. In some cases, conjugation of dienes with electron-withdrawing groups sufficiently deactivates them, avoiding DDQ interference.

Also, always be on the lookout for unique systems and reactivities!
The synthesis of sterepolide, an antibiotic fungal metabolite, exemplifies this concept in oxidative deprotections [2]. When using an excess of DDQ (8 equivalents), the authors found the allylic O-PMB over-oxidized directly to the ketone. In this case, this was convenient as it gave the target natural product, saving one step. However, this can complicate cases where we need deprotection only (most of the time).
Advanced Question: Special PMB Deprotection
As a twist on the previous information, you can ponder on this research [3]. Using 0.5 equivalents of oxalyl chloride led to efficient PMB deprotection of various substrates.

What could be a mechanism for this unique deprotection reaction?
Closing Remarks
PMB is a quite unique protecting group given the ability to remove it oxidatively (in addition to normal acid-mediated cleavage, not explicitly discussed here). In addition, PMB can also protect carboxylic acids, thiols, amines, amides… – or even phosphates! Basically, many other nucleophilic groups. The protection and oxidative removal make it a standard question in organic chemistry courses.
Learn more about other protecting groups, or watch my educational videos for advanced chemistry & science content!
PMB protection experimental procedure [4]
“To an ice-water cooled solution of SM (3.91 g, 15.2 mmol, 1 equiv) in THF-DMF (100 mL-30mL) was added NaH (2.43 g, 60.8 mmol, 4 equiv, 60% suspended in mineral oil) portionwise. After addition, the reaction was stirred at the same temperature until cease of gas releasing, then p-methoxybenzyl bromide (6.11 g, 30.4 mmol, 2 equiv) in THF (25 mL) was slowly added at 0 °C. The reaction mixture was stirred at 0 °C for 1 h, then quenched by slowly adding 1M solution of NaOMe in MeOH (15 mL). The reaction mixture was diluted with EtOAc (300 mL) and washed with water and brine. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo. The resulting residue was purified by silica gel flash chromatography (hexanes : EtOAc = 25:1-10:1) to give 5.28 g of product as a colorless oil in 92% yield.”
PMB deprotection experimental procedure [5]
“To a solution of SM (1.97 g, 3.95 mmol) in CH2Cl2:0.1 M pH 7 sodium phosphate buffer (18:1, 47 mL) at 0 °C was added 2,3-dichloro-5,6-dicyano-p-benzoquinone (1.17 g, 5.14 mmol) slowly as a solid. The reaction was warmed to rt and stirred for 1 h. The crude mixture was directly loaded onto a silica gel column with a top layer of MgSO4:sand (1:1, 0.5 inches). Elution with 5% to 30% EtOAc in hexanes yielded the product (1.45 g, 97%).”
PMB References
- [1] Oikawa, Y.; Yoshioka, T.; Yonemitsu, O. Tetrahedron Lett. 1982, 23, 885 | A facile chemoselective deprotection of the p-methoxybenzyl group
- [2] Barry M. Trost, John Y. L. Chung, J. Am. Chem. Soc. 1985, 107, 15, 4586 | Unusual substituent effect on a palladium-mediated cyclization: a total synthesis of (+/-)-sterepolide
- [3] Tetrahedron Lett. 2015, 56, 1080 | Facile and selective deprotection of PMB ethers and esters using oxalyl chloride
- [4] J. Am Chem. Soc. 2016, 138, 3926 | Total Synthesis of Mannopeptimycins α and β
- [5] Angew. Chem. 2018, 130, 16092 | Total Synthesis of Divergolides E and H
- P. Kocienski, Protecting Groups (Thieme)
- P. Wuts, T. Greene, Greene’s Protective Groups in Organic Synthesis (Wiley)






